Check out this awesome photo. More than likely this is a fall color image. But it’s still cool. Go Space!

This is a place holder for the upcoming school year.
This year Monegan is teaching four courses: AP Physics 1, General Physics, Astronomy and Algebra 1.
Physics and Algebra have their own pages. AP Physics 1 and Astronomy don’t . . . yet. But they will soon. Maybe even right now as you’re reading this.
Well, hopefully you’re starting to understand the idea of these gas
laws.
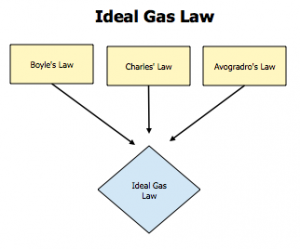
We’ve broken down the four laws we have used up to this point:
Boyle’s Law — PV = constant.
Charles’ Law — V = constant*T
Gay-Lussacs Law — P = constant*T
Avogadro’s Law — V = constant*n (n = number of moles)
When we put these all together, we get the Ideal Gas Law.
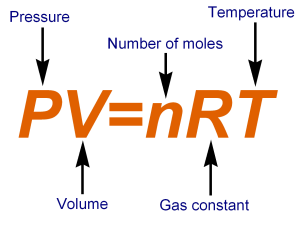
We’ll see how to use this law shortly, but it turns out be pretty easy.
If you’re still having some trouble with this, I’d recommend watching the video below. It moves quick, but it
‘s totally worth it.
Until next time.